
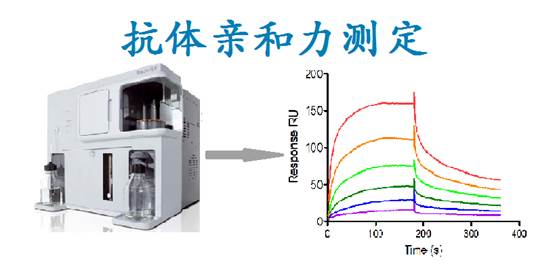
Introduction:
The binding force between antibodies and antigen-determining clusters is called antibody affinity, which is essentially a non-covalent force. It reflects the ability of antibody molecules and antigens to determine the binding of clusters. The determination of the affinity of antibodies to specific antigens is very important for screening antibodies. In general, the size of affinity can be represented either by the binding constant Ka or by the dissociation constant Kd. At present, the main method to determine the affinity between antibodies and antigens is to use BiaCore system to use surface plasma resonance (SPR) phenomenon to monitor molecular interaction in real time. Abrev adopts BiaCore X100 intermolecular interaction analysis system, which can provide efficient and sensitive antibody - antigen affinity analysis and provide important reference data for the screening of candidate monoclonal antibodies.
Competitive Advantages:
• Rich practical experience (Cheng Lin, Wu Xilin, et al. Mab, 2015) (Wu Xilin et al. JCI, 2018)
• High sensitivity and good experimental data
• Convenient and efficient, the price is favorable
Product/ Service details:
|
Product/ Service |
Customer provided |
Deliverable |
Turnaround Time (Business day) |
|
The antibody antigen was conjugated to the BiaCore chip |
Protein/antibody/small molecule (requirements are as follows) : Protein/antibody: > 0.2mg Fax: > 0.3mg Traverse buffer solution does not contain Tris (PBS buffer is recommended) |
Marathon affinity measurement report (including the combination of constant Ka, dissociation constant Kd and other data) |
3 - 10 Days |
|
The affinity between antibodies and antigens was determined by BiaCore X100, and Kd value was calculated by curve equation fitting |
Consulting Information:
Please contact us by email or telephone for the specific service price and content.
Email: xilinwu@abrevbio.com & bd@abrevbio.com
Phone: 18805158739