
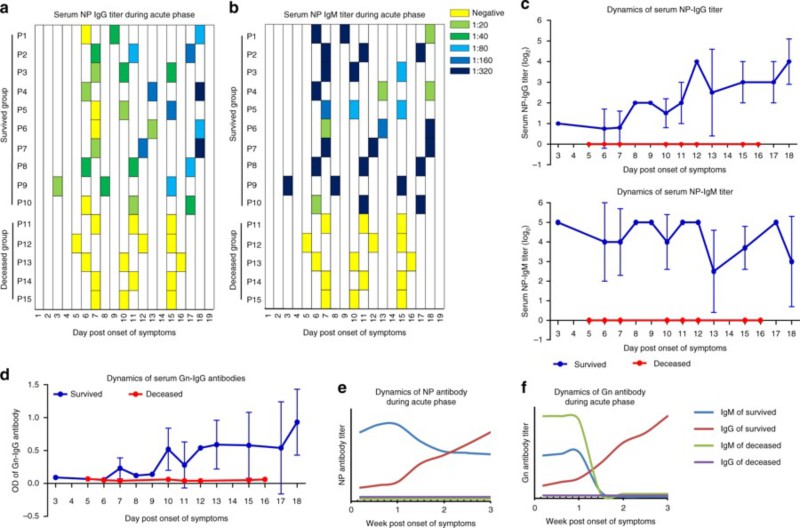
Fig. 1:SFTS antibody response in viral infection
(CD19In order to understand the mechanism of specific antibody immunoprotective deficiency, the composition and differentiation of B cell subsets in peripheral blood of SFTS patients in acute phase were dynamically analyzed. The results showed that the levels of Nave B cells (CD19+IgD+CD27-) and marginal zone-like B cells (CD19+IgD+CD27+) in the dead patients were significantly lower than those in the convalescent patients and the healthy controls. Both effector B cells, including plasmablast and memory B cells, were significantly increased in the convalescent and the dead groups, but unexpectedly. The plasma cell level of the dead group was higher than that of the convalescent group, suggesting that although B cells of the dead patients were activated in large quantities, no specific antiviral IgG antibodies were secreted for unknown reasons. This phenomenon is extremely rare in viral infection. Only a few cases of Ebola virus infection have been reported. In addition, the level of IgD-CD27- double negative B cells in the death group was also significantly higher than that in the rehabilitation group. At the same time, the results of IgM and IgG showed that the level of IgM-IgG + plasmacytes which achieved type-switching in the rehabilitation group of acute stage of SFTS showed a gradual upward trend, but was significantly lower in the death group, indicating that the virus-specific IgG secreted by the plasmacytes probably inhibited virus replication and provided immune protection for the infected persons. At the same time, it was confirmed that the loss of specific serum antibodies in the peripheral blood of the dead patients was due to the functional loss of the antibody secreting cells. Because of the presence of virus-specific IgM in the serum of some dead patients at the early stage of infection, it is speculated that the antibody secreting cells of the dead patients have failed in antibody type conversion. The expression of BLIMP-1, IRF-4 and XBP-1, which are important regulators of B cell antibody secretion, was also detected in the dead patients.
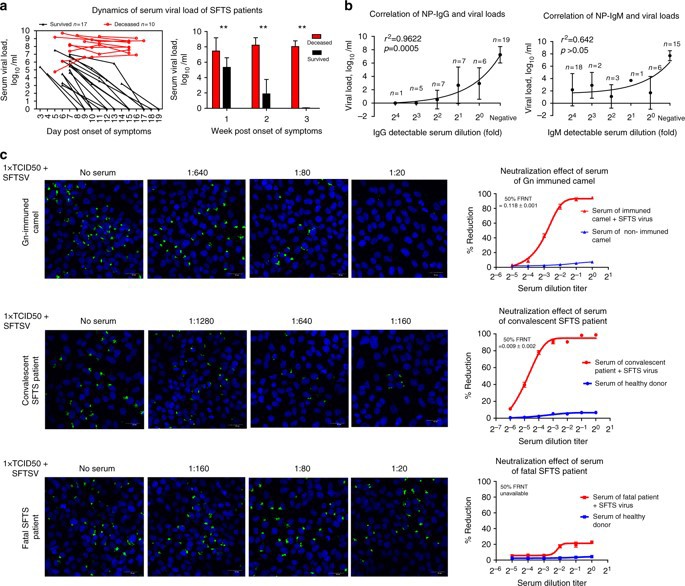
Fig. 2:SFTS production and biological function of antibodies in infection
In the early stage of SFTS, there were a lot of apoptosis and death in monocytes of peripheral blood of patients. Flow cytometry showed that the mDC in the rehabilitation group showed effective differentiation during the disease, while the number of mDC in the death group remained low, and the expression of costimulatory molecule CD86 remained low. Our in vitro experiments also found that SFTS virus-infected mDC significantly impaired the stimulation of T cells; mDC-infected T cells stimulated proliferation and cytokine secretion were significantly weaker than the control group. Therefore, we think that the impairment of mDC antigen presentation function plays an important role in the pathological progress of SFTS. Further, by dynamic analysis of follicular helper T cells (pTfh) in peripheral blood, we found that the number of pTfh in the first week after onset in the rehabilitation group was significantly higher than that in the death group. In vitro stimulation test, we found that the number of IL-21 positive pTfh in the rehabilitation group was significantly higher than that in the death group at the first and second week of onset, suggesting that effective pTfh differentiation occurred in the early stage of the disease in the rehabilitation group rather than the death group. Furthermore, the co-culture of Tfh cells and B cells also demonstrated that Tfh cells from rehabilitated patients could activate Naive B cells more effectively than those from dead patients, suggesting that dysfunction of Tfh cells from dead patients was a factor contributing to humoral immune dysfunction.
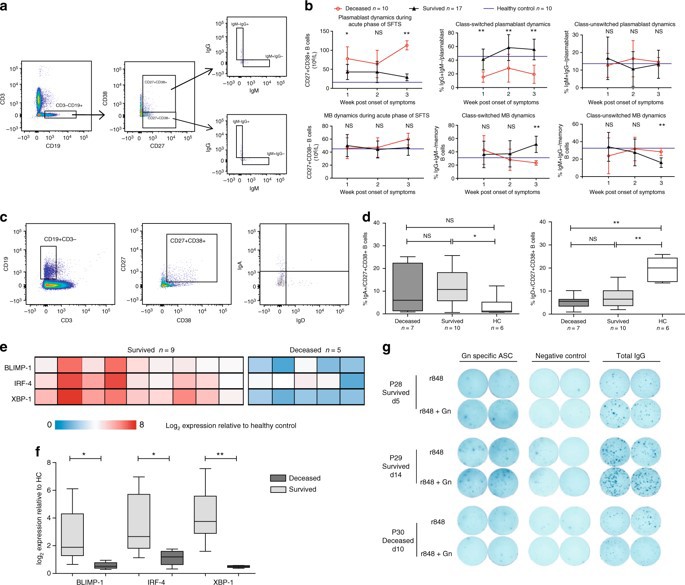
Figure 3: phenotype and function analysis of antibody secreting cells
Based on the above results, we can conclude that innate immune dysfunction and humoral immune dysfunction are important pathological mechanisms of fever with thrombocytopenia syndrome in the acute phase of new Bunia virus infection. The effective activation and differentiation of myeloid DC cells (mDC) and follicular Th cells (Tfh) play a key role in virus clearance. In the humoral immune response to SFTS, we found that the loss of Gn and NP-specific IgG antibodies was closely related to the outcome of death. The impairment of humoral immune response is the result of the overall disorder of B cell immune response, including the maturation defect of mDC, the impairment of MHCII antigen presenting function of mDC and B cells, and the failure of Tfh differentiation in early stage of disease. Current studies have pointed out the key role of Gn and NP specific IgG antibodies in the control of viral infection, and pointed out the direction of clinical diagnosis, prognosis and development of vaccine and therapeutic antibodies for SFTS.
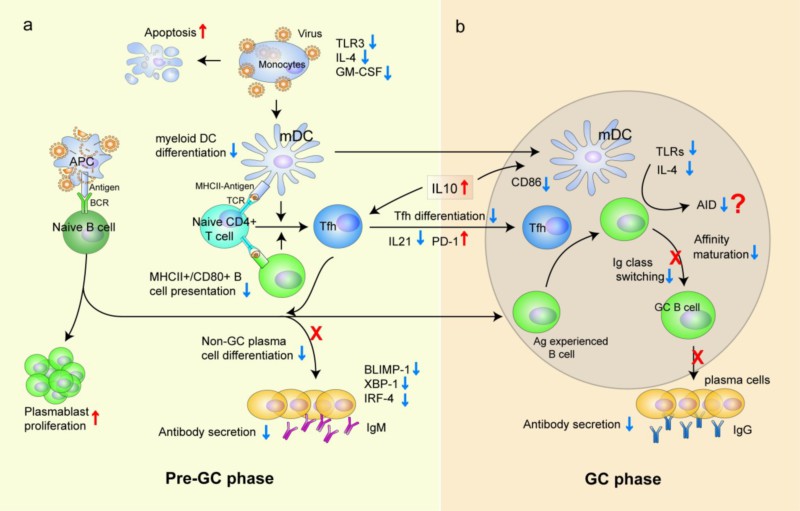
Figure 4:SFTS humoral immune deficiency in viral infection and its cellular and molecular mechanisms
The study was published on 20 August 2008 in Nature Communications 9:3328/DOI:10.1038/s41467-018-05746-9.Link https://www.nature.com/articles/s41467-018-05746-9, Deficient humoral responses and disrupted B-cell immunity are associated with fatal SFTSV infection, co-author of the paper Song Peixin, a doctoral student in the Department of nosocomial infection in the Gulou Hospital, and Zheng Nan, a medical college, are attached to the hospital. The correspondent author is Professor Wu Zhiwei, a medical college. Funding sources: major infectious diseases and national key R & D programs.